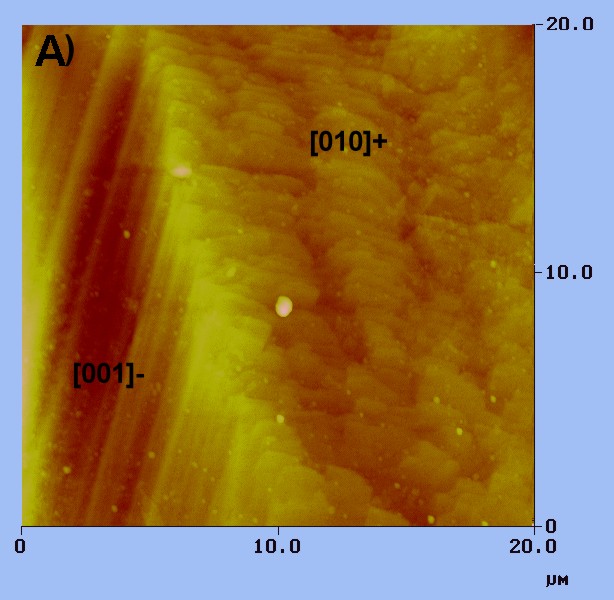
 This AFM topographic
image shows part of a growth hillock on the diopside face (100). Straight
steps parallel to the [001]- direction meet with wavy steps along the [010]
direction. The smallest steps detected are about 30 angstroms high.
This AFM topographic
image shows part of a growth hillock on the diopside face (100). Straight
steps parallel to the [001]- direction meet with wavy steps along the [010]
direction. The smallest steps detected are about 30 angstroms high.
Crystal growth can be studied:
Techniques applied include:
atomic force microscopy DIC microscopy scanning electron microscopy compositional mappping by electron probe microanalysis cathodoluminescence (to image compositional zoning) TEM on Pt-C replicas (with Hojatollah Vali) computer modelling of the geometry of zoning and crystal morphology using SHAPE

Hydrothermal diopside from the Orford Nickel Mine, near Brompton
(Quebec), is known for its unusual flattened morphology. Some natural crystals
have surfaces well enough preserved to show arrays of growth steps on the
(100) face. Kristy Thornton (kiki@eps.mcgill.ca) worked with me on
an undergraduate research project focussing on this mineral, and presented
her first results at GeoCanada 2000, (Calgary, June 2000).
 This AFM topographic
image shows part of a growth hillock on the diopside face (100). Straight
steps parallel to the [001]- direction meet with wavy steps along the [010]
direction. The smallest steps detected are about 30 angstroms high.
This AFM topographic
image shows part of a growth hillock on the diopside face (100). Straight
steps parallel to the [001]- direction meet with wavy steps along the [010]
direction. The smallest steps detected are about 30 angstroms high.
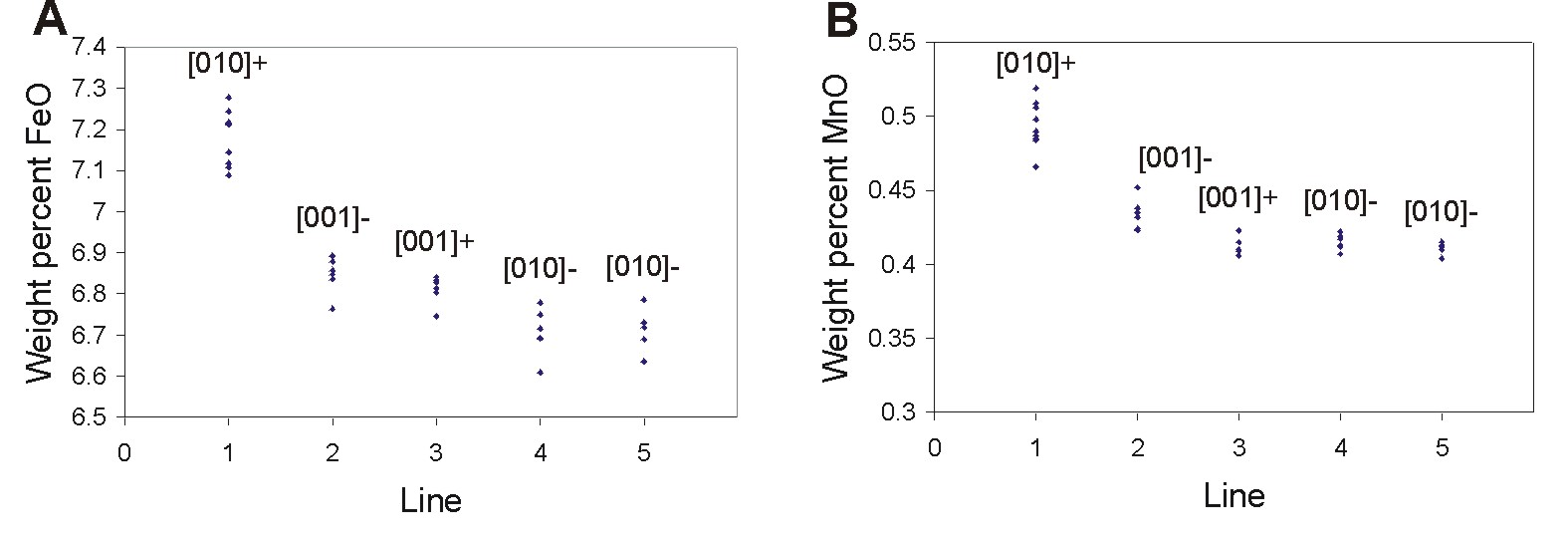
The 2 plots above show the small but systematic differences in FeO and
MnO concentrations detected by electron probe microanalysis among the
different portions of one growth hillock on the pyroxene surface.
The Fe and Mn content are always slightly higher on the wavy steps [010]+
than
elsewhere on any given growth hillock. The difference is not large
(about 0.5 wt. % FeO) but it indicates that the configuration of the cation
sites at the wavy steps enhances the incoporation of impurities.
I recently picked up some vesuvianite crystals from the Jeffrey
Mine (Asbestos, Quebec) and noticed striking V-shaped arrays of growth
steps on the larger prismatic faces. Vesuvianite offers a special challenge
for a student with a keen interest in some of the finer points of mineral
structure and chemistry. A few papers have discussed the lowering of symmetry
in some vesuvianite specimens but no one, to my knowledge, has examined
this from the point of view of its growth mechanism. Dissymetrization
is a phenomenon which had been related in some minerals to the relative
orientation of growth steps and face symmetry. This M.Sc. project would
involve a description of the surface microtopography by AFM, compositional
mapping of oriented sections through some crystals and an optical microscopy
study to look for evidence of a correlation between domains of lower symmetry
and the arrays of surface steps.
Calcite is my first love among minerals partly because of the stunning diversity of its morphology. Alfonso Mucci and I keep an eye for students willing to grow calcite crystals from aqueous solutions in the presence of all kinds of impurities. Mounir Temmam completed a Ph.D. thesis on the effect of Mn, Zn and Cd and of NaCl concentration on calcite morphology (Temmam et al., 2000, and two more manuscripts in preparation and review). Kristy Thornton will now tackle the effect of Pb on calcite growth for her M.Sc. project, and pursue work initiated by Jody Vaillancourt (undergraduate project, 1995).
Al and I also team up with Hojatollah Vali who trains some of our students in the preparation of Pt-C replicas of their surfaces. This approach can be used on microcrystalline powders to image by transmission electron microscopy (TEM) the changes in crystal morphology induced by changes in the growth medium on crystallites that are barely 5-20 µm across.
Trace elements in natural calcite are often intricately zoned,
but the interpretation of zoning patterns is a frustrating exercise without
a basic understanding of the relationship of crystal morphology. Students
interested in carbonate diagenesis can get quite an education in the interpretation
of 2-D zoning patterns by sectioning natural calcite crystals and reproducing
the zoning patterns visible in cathodoluminescence using the software SHAPE.
Natural barite shows fairly diverse habits, commonly with sector
zoning. Some gem-quality specimens preserve interesting arrays of growth
steps, particularly ontheir {102} and {001} faces. Luc Rock (1997,
undergraduate project) documented step-specific incorporation of Sr on
(102) as well as sector zoning of Sr among non-equivalent faces on a specimen
donated by Dr. Peter Richards. M.Sc. student Peggy Varga extended this
work to a suite of barite crystals from 4 other localities (obtained from
the Canadian Museum of Nature) and mapped in each one the distribution
of Sr on sections polished parallel to the (001) face.
One of the most intriguing observations so far has been the
patchy zoning of Sr within {102} and {001} sectors, and whose geometry
clearly correlates with the different orientations of steps on the as-grown
surface. This type of intrasectoral zoning
suggests that layers nucleated at and spread out from specific surface
sites, in the typical fashion of spiral growth. Yet, Pina et al.
(1998: see Nature, 395, 483-486) dismissed the possibility of spiral
growth on barite on the basis of their experimental observations and theoretical
calculations. Are these natural barite crystals trying to tell us that
the spiral growth mechanism is possible in barite, but under conditions
not yet reproduced in laboratory?